Abstract
INTRODUCTION
We have previously described AUTO1, a CD19 CAR with a fast off-rate binding domain, designed to reduce CAR T-cell immune toxicity and improve engraftment. Clinical testing in two academic studies in relapsed/refractory (r/r) paediatric [NCT02443831; CARPALL] and adult B-ALL, B-NHL and B-CLL [NCT02935257; ALLCAR19] confirmed the intended function of the receptor, with low levels of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) and long-term engraftment of CAR T-cell 1,2. Based on data in adult B-ALL, we initiated a phase Ib/II registration study in r/r adult B-ALL [NCT04404660; FELIX]. To facilitate this study and future commercialization, industrialization of the manufacturing process was required.
METHODS & RESULTS
The original process in the academic studies was based on the Miltenyi CliniMACS Prodigy T cell transduction process. Leukapheresis was performed at the same site as manufacture. T cells were isolated from pheresis by CD4/CD8 positive selection and seeded onto the Prodigy to be activated using the Miltenyi CD3/CD28 targeting activating reagent, TransAct. The following day, transduction was carried out using a lentiviral vector. Cells were cryopreserved after an expansion phase of up to day 10 of the process.
To facilitate industrialization of the AUTO1 manufacture in the multi-center, multi-regional FELIX study, we first explored the use of cryopreserved pheresis (81.3% median viability pre-selection (range 71.9 - 94.3), 1.0 days median doubling time (range 0.9 - 1.5) and 47.6% median CD19 CAR expression (range 19.1-62.1)). We concluded that optimal manufacture includes the use of fresh pheresis and the initiation of manufacture within 72 hours (99.0% median viability pre-selection (range 92.5 - 99.7), 1.3 days median doubling time (range 1.1 - 2.1) and 69.3% median CD19 CAR expression (range 22.9-86.2)).
To further simplify the process, we explored removal of the pre-selection step. Full-scale runs using starting material from 4 healthy donors were conducted to compare CD4/CD8 selected with unselected cells. On the day following activation, selected cells displayed a higher percentage of viable cells, defined as cPARP-FVS780- (median: 76.1%, range: 84.5-66.4) as compared to unselected cells (median: 52.2%, range: 43.6-59.0). In addition, selected cells demonstrated a median of 23-fold expansion (range: 20.0 - 29.1) compared to a 13.3-fold expansion for unselected cells (range 6.1-17.4). Median transduction efficiencies of viable CAR+ T-cells were 53.9% (range: 43.2-56.9) and 78.0% (range: 64.5-81.1) in selected and unselected cells, respectively. CD4/8 pre-selection was determined to be a critical part of the process.
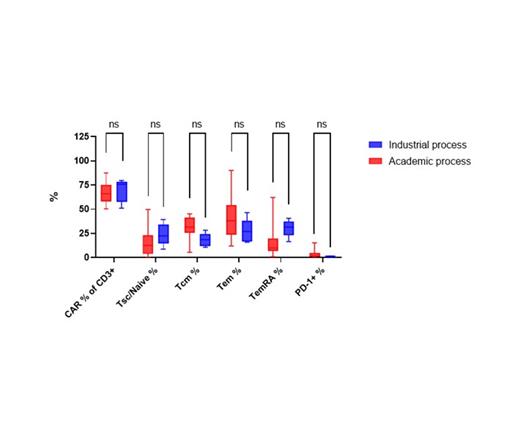
A comparison of phenotype between 18 batches manufactured using the academic process and 5 batches produced from fresh material using the industrial process was carried out. No significant differences, as determined by 2-way ANOVA, were observed between the percentage of CAR+ CD3+ cells, the memory phenotype (% TSC/naive, % TCM, % TEM and % TEMRA) and the percentage expression of PD1 (figure 1). The CD4/CD8 ratio was also comparable between products of the two processes.
Data from the initial 6 fresh in patients show that engraftment in the FELIX study is consistent with ALLCAR19 engraftment results. Additional patients, updated clinical data and longer follow-up will be presented at the conference.
CONCLUSION
Industrialization of an autologous Miltenyi CAR T process is feasible, leading to a comparable product to that manufactured in an academic setting. We have now opened the pivotal multi-center phase II part of the FELIX study in r/r adult B-ALL patients.
REFERENCES
Ghorashian S et al. (2019) Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med, 25(9):1408-1414.
Roddie C et al. (2021) Durable responses and low toxicity after fast off-rate CD19 CAR-T therapy in adults with relapsed/ refractory B-ALL. J Clin Oncol, in press
Figure 1. Comparison of phenotype between 18 CAR T cell batches manufactured using the academic process and 5 batches produced using the industrial process. Boxes represent median, 25th and 75th percentiles and whiskers represent minimum and maximum.
Culshaw: Autolus Ltd.: Current Employment. Arce Vargas: Autolus Ltd.: Current Employment. Santiago Toledo: Autolus Ltd.: Current Employment. Roddie: Novartis: Consultancy; Celgene: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau. Shaughnessy: BMS: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Kite: Honoraria, Speakers Bureau. Cerec: Autolus Ltd.: Current Employment. Duffy: Autolus Ltd.: Current Employment. Perna: Autolus Ltd.: Current Employment. Brugger: Autolus Ltd.: Current Employment. Merges: Autolus Ltd.: Current Employment. Pule: Autolus Ltd: Current Employment.